The DMS
e – Document Management System
Discover a tailored Document Management Solution built on the robust Mayan e-DMS platform, specifically designed for the pharmaceutical industry. Our system ensures seamless document creation, management, and compliance with industry standards like FDA 21 CFR Part 11. Enhance your operational efficiency with secure, version-controlled workflows, electronic signatures, and customizable templates—all in one intuitive interface.
Seamless Document Creation & Upload
Upload, Create and manage documents directly within the system using an integrated WYSIWYG editor, complete with customizable templates for standard operating procedures, reports, and more.
Regulatory Compliance
Built-in adherence to FDA 21 CFR Part 11 and other industry standards ensures your documents meet all necessary regulatory requirements, including electronic signatures and audit trails.
Advanced Workflow Automation
Streamline your document approval and review processes with automated workflows, ensuring that every document passes through the right channels for timely and accurate sign-off
Secure Access Control
Implement fine-grained access controls and permissions to ensure that only authorized personnel can view, edit, or approve sensitive documents, protecting your data’s integrity.
Version Control and Audit Trails
Track every change with robust version control and maintain detailed audit trails to ensure full traceability and accountability for all document actions.
Scalable Integration
Easily integrate with existing enterprise systems like ERP, LIMS, and PLM to create a unified environment for managing all your pharmaceutical documentation needs.
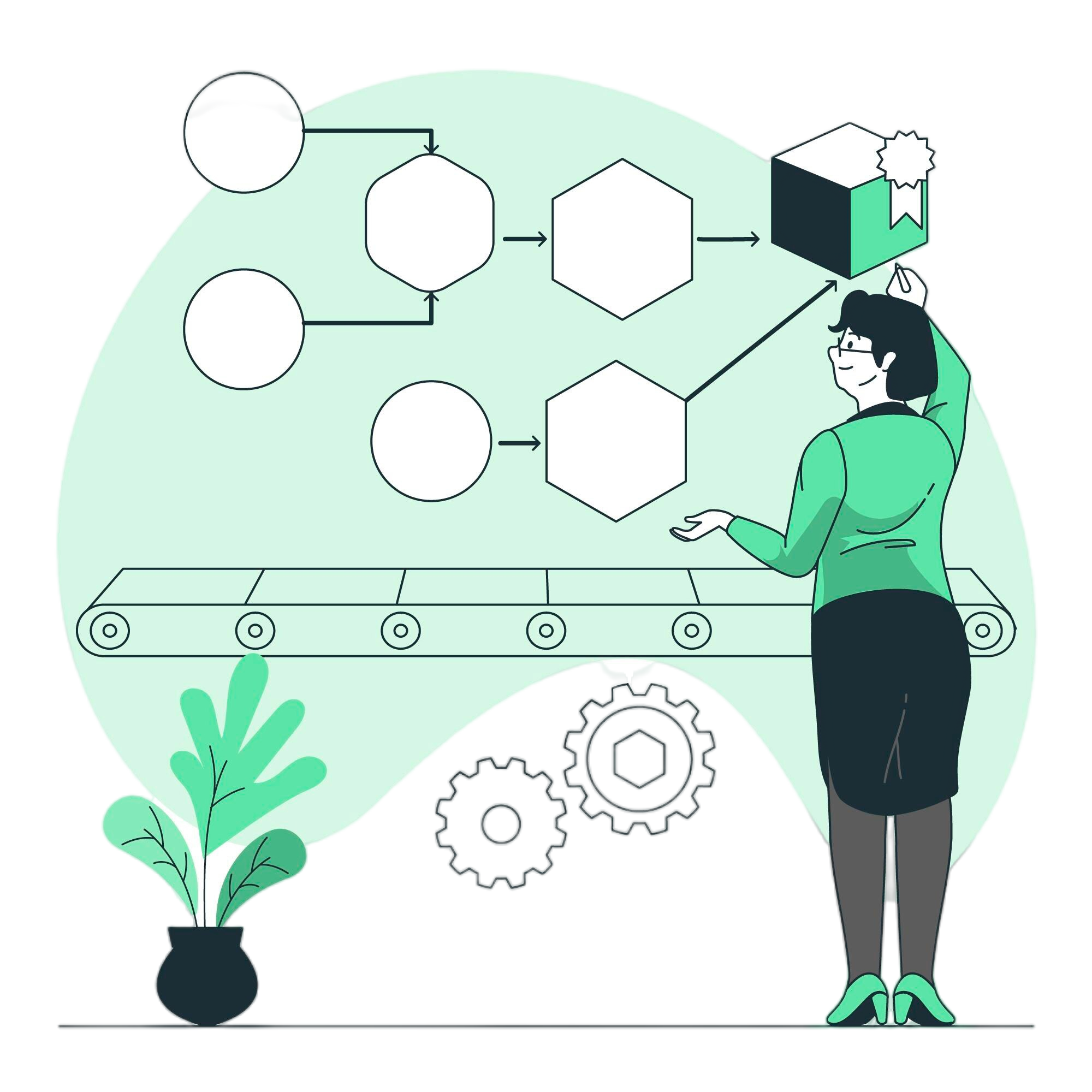
DMS General Workflow
Document Creation & Upload
Easily create, upload, and organize documents in a centralized, secure platform.
Review Process
Streamline reviews with automated workflows and clear stakeholder assignments.
Approval Process
Ensure compliance with structured, multi-level approval pathways.
Version Control and Publishing
Maintain accurate records with full version tracking and controlled publishing.
Distribution
Share documents securely with teams, clients, or departments as needed.
Periodic Review and Audit
Keep content updated with scheduled reviews and audit trails.
Archival and Disposal
Manage document lifecycle with safe archival and compliant disposal processes.
